As a pharmaceutical manufacturer, distributor, or importer in Nigeria, it’s important to understand NAFDAC’s (National Agency for Food and Drug Administration and Control) traceability policy. The primary goal of NAFDAC is to safeguard the health of the population and prevent the distribution of fake and substandard medicines and other regulated products. According to the WHO, this is still a huge problem in sub-Saharan Africa.
In June 2019, the Federal Ministry of Health & NAFDAC brought together stakeholders to chart the path for the implementation of traceability in Nigeria. These stakeholders, which included the private & public sector partners, put together the vision, strategy, and roadmap for its implementation.
Their aim? Use global standards to ensure that the pharma industry in Nigeria:
- Provides visibility of product status from plant to patient;
- Promotes trust in the pharmaceutical sector and healthcare system;
- Provides an increased opportunity for the trade of domestically manufactured pharmaceuticals;
- Increases quality of data to support pharmacovigilance;
- Decreases the presence of substandard and falsified (SF) medications;
- Enables efficiencies across the supply chain; and ultimately,
- Increases patient safety.
To achieve these outcomes, the group resolved that all pharma stakeholders would have to cooperate closely to achieve 5 strategic objectives:
Strategic Objective 1: Establish a governance structure to lead the strategy, advocacy, collaboration, resource mobilization, and oversight of global standards and traceability implementation.
Strategic Objective 2: Strengthen the regulatory environment to include legal frameworks that enable the traceability of quality pharmaceuticals through the legitimate supply chain.
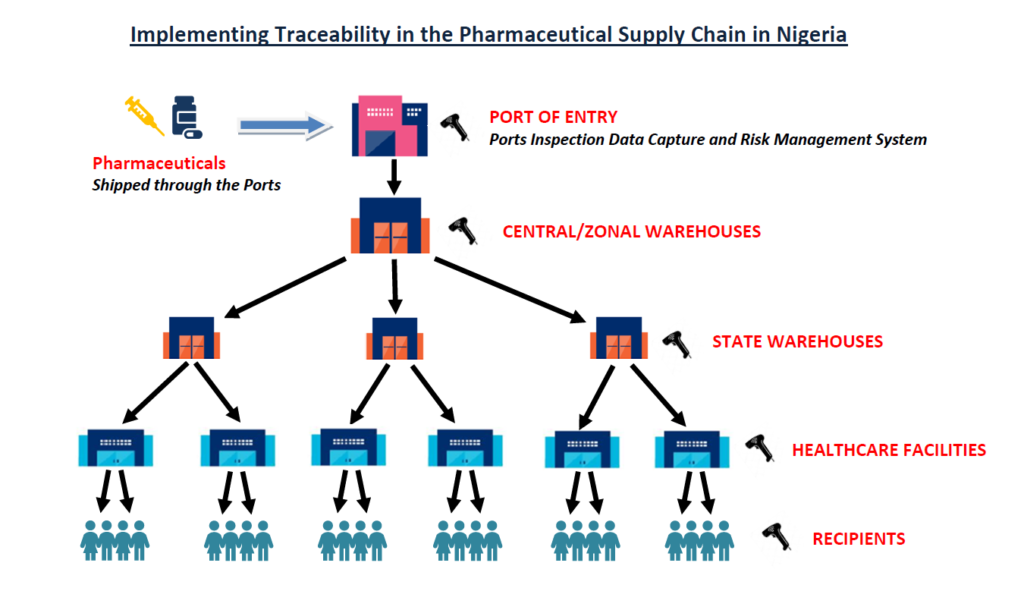
Strategic Objective 3: Create efficiencies in the public and private health supply chains through standardized identification, automated data capture, and reporting.
Strategic Objective 4: Build and sustain technology to support the interoperability of health information systems and implementation of traceability to improve data visibility.
Strategic Objective 5: Enable the use of standards to support the identification and authentication of commodities dispensed to end-users at service delivery points in the public and private sectors.
In 2020, NAFDAC announced the launch of the traceability strategy and outlined a 5-year plan for its implementation. Meaning, by the end of 2024 pharmaceutical stakeholders will have all been onboarded on the regulator’s traceability system.
The years following were met with some pushback and complaints about the complexity and cost of implementation but with support from bodies like USAID, GS1, and many others, the regulatory body remains steady in its push to implement the traceability strategy by the given timeline.
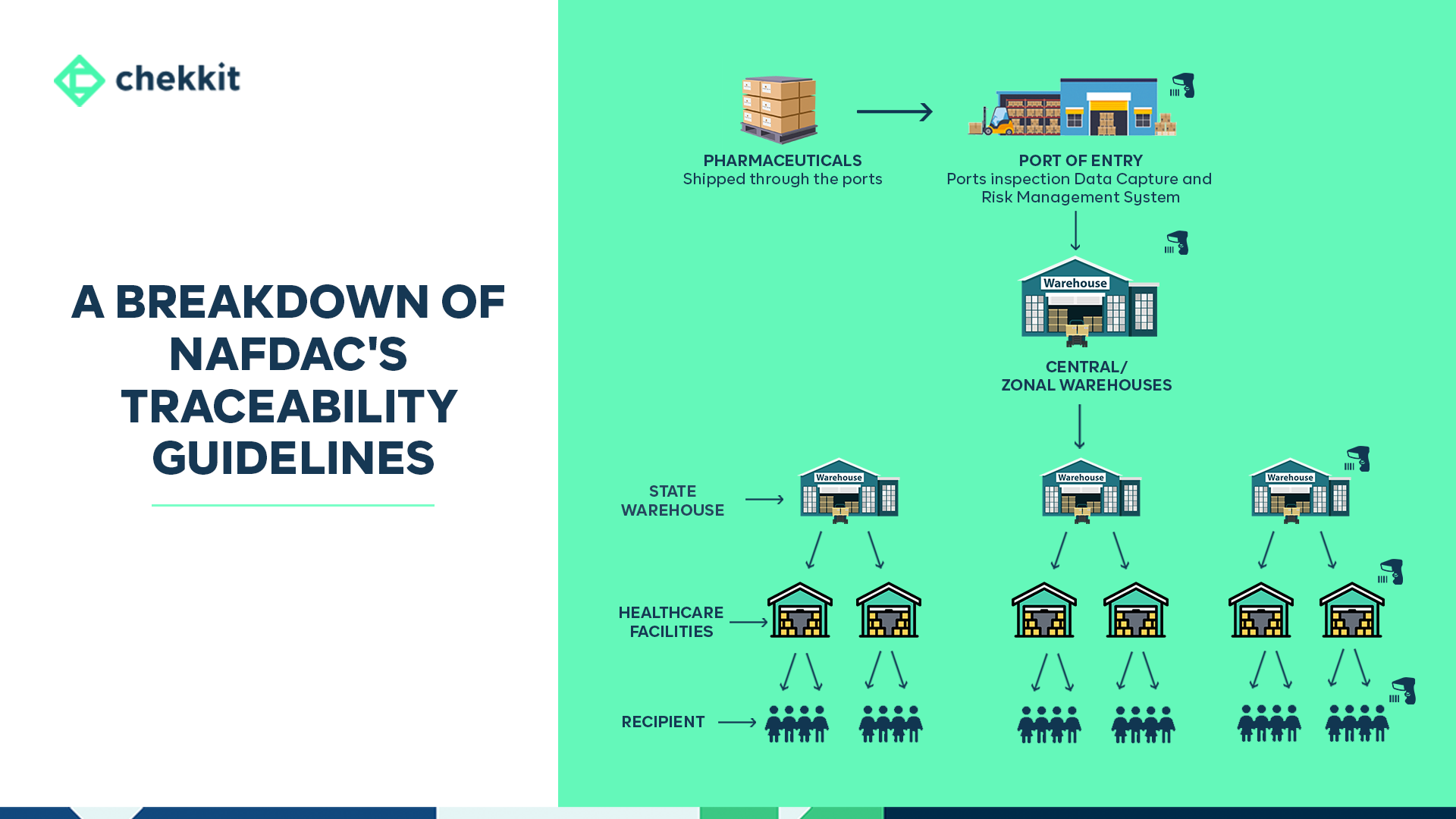
How Will The NAFDAC Traceability System Work?
For any traceability system to work, there has to be a flow of data about that product as it moves from manufacturer to patient. A traceability system maintains the flow of data about the product, including the master, transaction, and event information related to an item in the supply chain.
Working with standards and guidelines from GS1, pharmaceutical products at different packaging levels will be required to carry unique identifiers (which can be bar codes or RFIDs) that hold information on the creation and movement of that product. To get a very sound understanding of this standard, please read our article on the GS1 EPCIS standard.
Traceability systems generally take on one of three models — verification, track & trace, or both. While NAFDAC is looking to implement both models, this will likely be done in phases.
Phase 1: Verification Model
A verification model refers to checking at a single point in the supply chain that the identification data held in the barcode is assigned by the manufacturer of the product. Because verification is technically more straightforward, this is typically the ideal first phase.
Here, only the primary or secondary package might be required to carry a unique code.

Phase 2: Track & Trace Model
A track and trace model supports the capture of data from trading partners as the product moves through the supply chain, from the manufacturer to the end user. This will require serialization across all sellable packaging levels and the easy sharing of data across supply chain stakeholders.

In addition to the traceability model, NAFDAC will also work closely with GS1 to maintain its central repository (database) of all the pharmaceutical products (and their master data) being distributed in Nigeria. In this centralized traceability model, event data from all supply chain parties are stored in NAFDAC’s repository.
The repository will serve as the hawk eye monitoring everyone and making sure we all play by the rules.
How Can Stakeholders Comply with NAFDAC's Traceability Guidelines?
To comply with NAFDAC’s guidelines and the GS1 standard, stakeholders like manufacturers & importers will have to work with GS1 solution providers in Nigeria. These are the technology companies building software solutions that use the data-sharing standards of GS1. To learn more, please read this article on how to work with a GS1 solution provider

Here Are Some Other Resources You Will Find Useful:
1. 5 Most Important criteria when choosing your pharma serialization software
2. How to stop market diversions with Traceability
3. How to stop inventory stockout of medicines using traceability
4. How to fight counterfeit drugs using traceability
5. The 2 best ways to serialize your pharmaceutical products
If you’re interested in learning how you can easily comply with pharma regulations in over 100 countries globally without breaking the bank, we will be more than happy to walk you through our technology.
As a pharmaceutical manufacturer, supply chain manager, importer, or distributor, this technology will help you stay ahead of the curve and ensure that your products reach patients safely and efficiently.