Before we go into how you can implement serialization for your pharmaceutical products, let’s do a recap of what serialization is and why it is important.
You might end up realizing that you don’t need serialization after all.
What is serialization in pharmaceutical packaging?
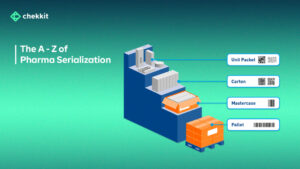
Pharma serialization is the process of assigning unique serial numbers to every drug packaging that is sellable; primary, secondary, and tertiary.
So from blisters to bottles, cartons, and pallets, they all carry a unique identifier. This unique identifier can either be QR Codes or DataMatrix Codes (depending on the country/region) and carry information about the product’s origin and validity.
These codes are then printed visibly on the respective packaging and are linked to each other. That is, the code of a bottle of pills is linked to the code of the carton it came in, which is also linked to the pallet that transported it from the factory/warehouse.

What are the benefits of serialization in the pharmaceutical industry?
Serialization for traceability became needed in the pharmaceutical industry mainly due to counterfeits. Packaging serialization achieves this by creating a closed system where the distribution of medicines can be controlled and monitored by stakeholders like regulators, manufacturers, distributors, healthcare providers, and even patients.
Over the years though, the benefits of implementing serialization have spread to other parts of the business. Here’s a summary of some of the most popular ways stakeholders have benefited from the serialization of pharmaceutical packaging:
1. Improve your supply chain & distribution to stop losses
Your supply chain becomes more efficient and data-driven. You can easily manage inventory, track assets in transit, and monitor things like temperature as your products are transported. In essence, you have a 360-degree view of your products as they move through the supply chain.
2. Stop the spread of counterfeits & regain lost market share
Serialization empowers all stakeholders in the supply chain to be able to verify the authenticity of the products they receive. This also applies to the patient who walks into a pharmacy to buy medicine. There’s also the pharmacovigilance aspect that monitors these verifications for signs of suspicious activities so that distributors of counterfeits are nabbed.

3. Automate the process of product recalls and make it more efficient
Say for example there’s been a problem with a batch of products or they need to be recalled for any other reason. The traceability that serialization offers helps you identify where the products are and automatically start and monitor the process of getting them back. Serialization also ensures that these returns are efficiently tracked for accounting purposes.
4. Engage last-mile patients directly & personalize your marketing communication
The marketing guys are not left out because by serializing the packaging of the drugs that patients come in contact with, you can engage them and start building that personalized relationship that leads to brand loyalty. From sharing information to engaging them with exciting activities, pharmaceutical brands can start adding that human element to their brands.
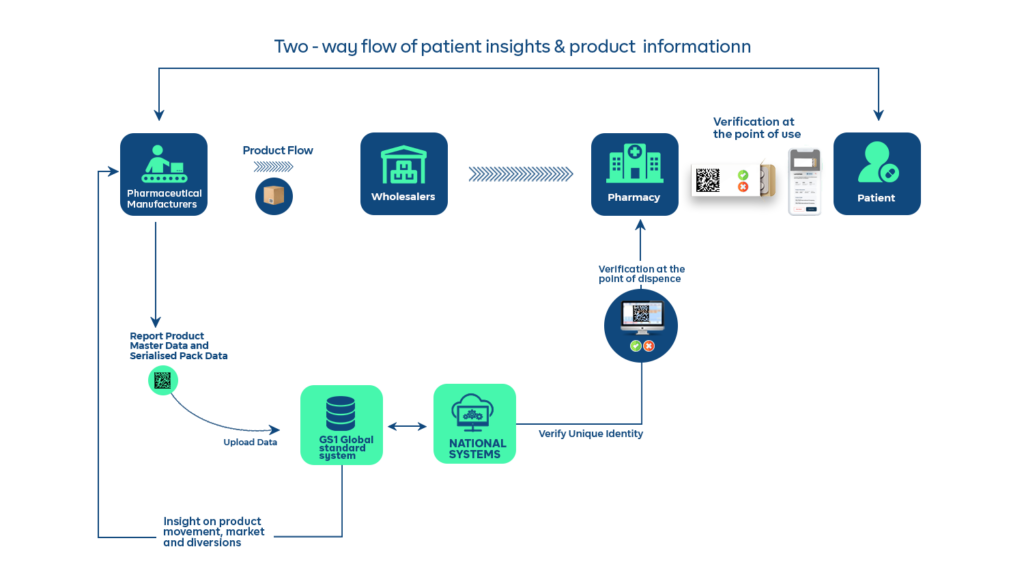
5. Get access to big data and insights on your products, market, and last-mile patients
Serialization of pharmaceutical packaging is a game changer for data collection and analysis. Why? Because it gives manufacturers access to not just data on the movement of their products but also insights into who their patients are, where they are, and why they buy. Even better, you get access to millions of data points about everything from your products, to your market, your distribution partners, and finally your consumers. This is the kind of big data that Artificial Intelligence can work magic with and start giving you accurate predictions.
Just in case these benefits aren’t enough you whet your appetite, remember that serialization in pharmaceutical packaging is now a regulation in over 100 countries globally. You will have to do it sooner or later if haven’t implemented it already.
What are the most commonly used methods for serialization in the pharmaceutical industry?
Now that you know what you can gain from serialization, the next big question is;
How can I get my products serialized?
There are two popular ways currently being used by many pharmaceutical manufacturers based on criteria like cost, regulatory compliance, complexity, efficiency, etc. We’ve done a comparison below:
1. In-Line Serialization of Pharmaceutical Packaging
As the name implies, in-line serialization means printing the serialized codes on the pharmaceutical product’s packaging directly on the production line. Everything automated.
As the codes are being printed, they are scanned to activate that product and then automatically tagged with the serialized codes on the boxes they’re grouped in. This method is what’s currently used by multinational pharmaceutical companies globally.
Pros of In-Line Serialization
- There is zero room for error and it can automatically manage all levels of serialization
- Once implemented, it doesn’t slow down your production or operations processes
Cons of In-Line Serialization
- It is expensive to implement as new production machinery will be required for manufacturers without such automation in their production lines already.
- It takes more skill to manage and troubleshoot any issue that might arise during use. Training and retraining workers will be likely required
2. Label Serialization of Pharmaceutical Packaging
If we’re being honest, you will agree with me that many of the small to medium-scale producers of pharmaceutical products do not have fully automated production lines.
To implement serialization, they can use sticker labels that can be applied to their products automatically (by extending their production lines with a labeling machine) or semi-automatically (by applying with a hand-held labeling device).
This method is also very beneficial for distribution companies that would like to implement their own closed ecosystem or even for NGOs and donor bodies that fund programs around medicine distribution.
Pros of Label Serialization
- It is more affordable to implement
- It can be implemented in days as opposed to in-line serialization
Cons of Label Serialization
- The human element means there’s always room for error
- The more levels of packaging that need to be serialized, the harder it gets to use this method
- Will slow down your production and operation process as this is a new process that requires some form of manpower.
How to choose a serialization software for your pharmaceutical packaging
Regardless of the method of serialization you decide to use for your pharmaceutical products, the most important aspect is that you get the software you use spot-on.
Why? This is what ensures you’re able to get the most value from your serialization efforts. After all, why settle for half when you can have it all?
Here are 5 important things to consider when picking a pharma serialization software.
Chekkit’s anti-counterfeit solution and serialization solution creates unique QR codes or DataMatrix codes for all your packaging levels. Our unique verification process helps you collect insights on drug efficacy, market diversions, side effects, helping you stay a step ahead of the competition always.
Will you like to see how it works? Get a free product demo