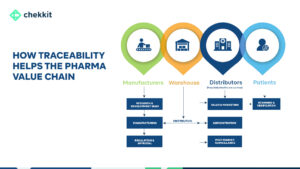
The pharmaceutical industry is under intense pressure to maintain high standards of product quality, safety, and efficacy. Traceability is an essential component of ensuring product quality and safety, especially in the case of drugs. However, implementing a traceability system can be a challenging process.
This article will examine the biggest challenges faced by pharmaceutical manufacturers when implementing a traceability system and discuss practical solutions that can be used to mitigate them.
The 3 biggest challenges with implementing supply chain traceability of medicines
Challenge 1: Difficulty in getting buy-in from distributors and retailers
This is because the implementation of a traceability system can affect the existing distribution and supply chain practices. Without getting the buy-in from distributors and retailers, you will never truly track and trace medicines end to end.
How to get buy-in from distributors and retailers
1. Work with their current processes as much as possible
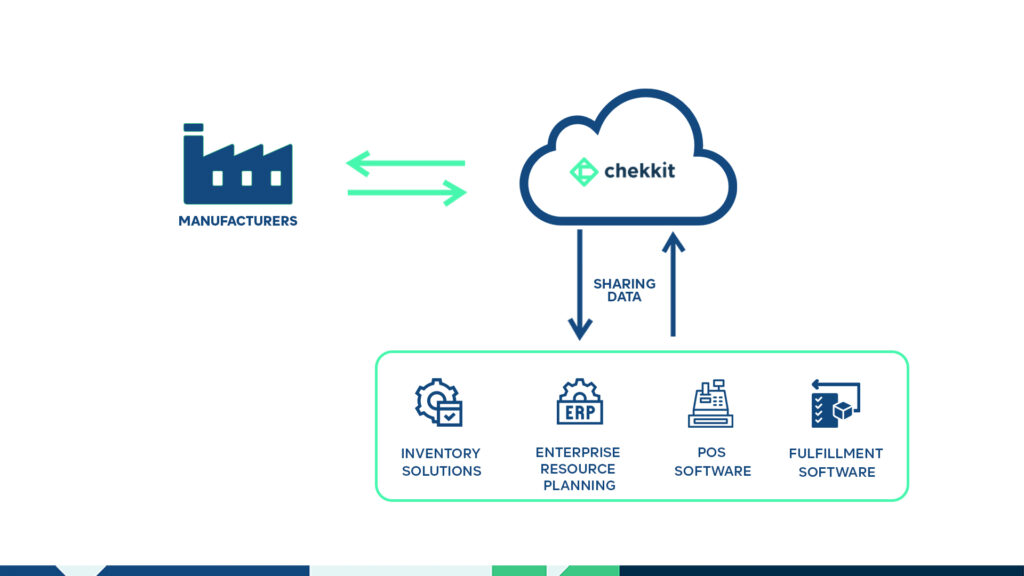
Many of your distributors and retailers already have tools they use in their day-to-day operations. For example, if you can automatically pull data from the inventory management software being used in your distributor’s warehouse with little to no human input, the distributors won’t need to worry about any obstruction to their processes.
This is one of the reasons Chekkit’s traceability tool has been effective over the years. You can now share data efficiently across all your supply chain partners in just days as opposed to many months of failed back and forth.
2. Involve distributors and retailers in the implementation process
Manufacturers can involve distributors and retailers in the implementation process by collaborating with them to develop a traceability system that works for everyone. By involving them in the implementation process and also providing adequate training programs, distributors and retailers will feel like they have a stake in the project, making it easier to obtain buy-in. You can also offer incentives to distributors and retailers who comply with the traceability system, such as better pricing, host raffle draws, or other benefits.
Again, Chekkit does this perfectly with rewards tied to different supply chain events.
Challenge 2: The high cost of serialization & having to modify your production line
Pharmaceutical manufacturers often face the challenge of having to change their production lines and processes to implement a traceability system. This can be a costly and time-consuming process, and manufacturers may be hesitant to make changes to their existing production lines and processes.
How to reduce the cost of serializing your products
1. Serialize your products after production
This typically involves attaching serialized stickers to your products. This can be done in your warehouses by using hand-held labeling devices which cost about the same as a car battery. This is a game changer for pharmaceutical manufacturers, especially in developing countries where resources are limited.
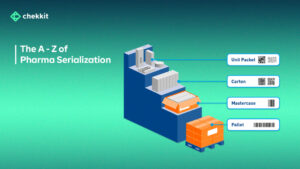
Chekkit enables you to serialize all packaging levels from primary to tertiary using this method and also save close to 70% on costs.
2. Implement a modular traceability system
This allows you to upgrade your existing production lines and processes in phases. This approach can reduce the upfront cost of implementing the traceability system and enable you to spread the cost over a more extended period. You can also choose to implement the traceability system in stages, starting with the most critical products, and then gradually expanding the system to cover all products.
For more ways to reduce costs, read this article on how to implement traceability in a cost-effective way.
Challenge 3: Unskilled personnel to manage & maintain the implementation
Implementing a traceability system requires specialized knowledge and skills, such as understanding the regulatory requirements for serialization and track-and-trace, configuring and maintaining the necessary software and hardware systems, and training personnel to use the system effectively. However, you probably don’t have the personnel with the necessary expertise and experience to do all that.
How to close the traceability management knowledge gap
1. Partner with reliable third-party providers
Traceability solution providers like Chekkit have the necessary expertise and experience to configure and maintain the necessary systems, train personnel, and ensure compliance with regulatory requirements. You can also collaborate with industry associations and trade organizations that specialize in traceability and serialization. These organizations can provide guidance and support on implementing a traceability system, as well as connect manufacturers with skilled personnel and GS1 solution providers
2. Invest in training and education:
Pharmaceutical manufacturers can invest in training and education for their personnel to develop the necessary skills and knowledge for implementing and managing a traceability system. This can include in-house training, attending conferences and workshops, or enrolling in online courses.
Chekkit organizes regular webinars to help supply chain professionals stay up to date with the recent happenings in the world of pharmaceutical traceability.
3. Engage with regulatory bodies:
You should always engage with regulatory bodies and agencies to ensure that you are aware of the latest requirements and standards for traceability and serialization. This can help help you to design and implement systems that are compliant with the relevant regulations and also ensure your opinions are taken into consideration for subsequent regulations.
Without a doubt, implementing a traceability system in the pharmaceutical industry is vital to ensure product quality and safety but sadly, it comes with its fair share of very tough challenges. However, the good news is that practical solutions exist to mitigate these challenges. Companies like Chekkit have already proven to be reliable partners in the pharmaceutical industry’s quest for improved traceability, and by following their lead, we can create a safer and more transparent pharmaceutical supply chain for everyone.
If you’re interested in knowing how our pharma serialization and traceability software for manufacturing works, we will be more than happy to walk you through our technology.
As a pharmaceutical manufacturer, this technology will help you stay ahead of the curve and ensure that you can spot new market opportunities long before the competition.