I was having coffee with a friend in Brussels and after listening to me talk passionately about how chekkit is helping the world get rid of fake products, he simply said he doesn’t see why he would need an app to check for fake medicines. To him, these weren’t problems of the west but of developing nations.
While he was wrong, as there are still many recorded cases of counterfeits in the west, he was also right that the problem is more keenly felt in continents like Africa, Asia, and parts of South America.
Why are counterfeit drugs still a problem in many developing regions?
Counterfeit medicine remains a significant problem in many African countries due to a range of factors, including weak regulatory frameworks that fail to effectively monitor the production, distribution, and sale of drugs, poverty that forces people to buy cheap drugs from unregulated sources that turn out to be fake, limited public awareness of the dangers of counterfeit medicines, corruption that allows unscrupulous individuals to take advantage of weak regulatory frameworks to produce and sell fake drugs, and inadequate enforcement mechanisms that make it difficult to track down and prosecute those involved in the production and sale of counterfeit medicines.
To tackle counterfeits on a large scale, two things need to be put in place:
- A way to make the distribution of drugs as restricted as possible and cut out unauthorized players from the supply chain.
- An easy way to differentiate between the original and counterfeit versions of a product.
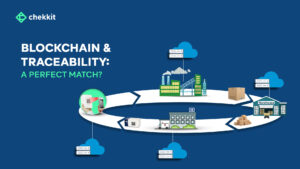
In this article, we’ll discuss how serialization and traceability solutions can help achieve both and provide extra value for all players in the pharmaceutical supply chain.
How Does Pharma Serialization Help in Identifying Counterfeit Drugs?
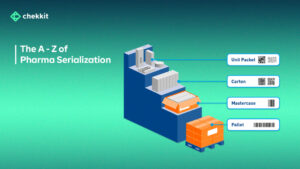
What is serialization in the pharmaceutical industry? Serialization in the pharmaceutical industry involves assigning a unique code to each individual package or unit of medicine, which is then tracked throughout the supply chain. The unique code can be in the form of a barcode or a 2D matrix code, and it contains information about the product, such as its batch number and expiry date.

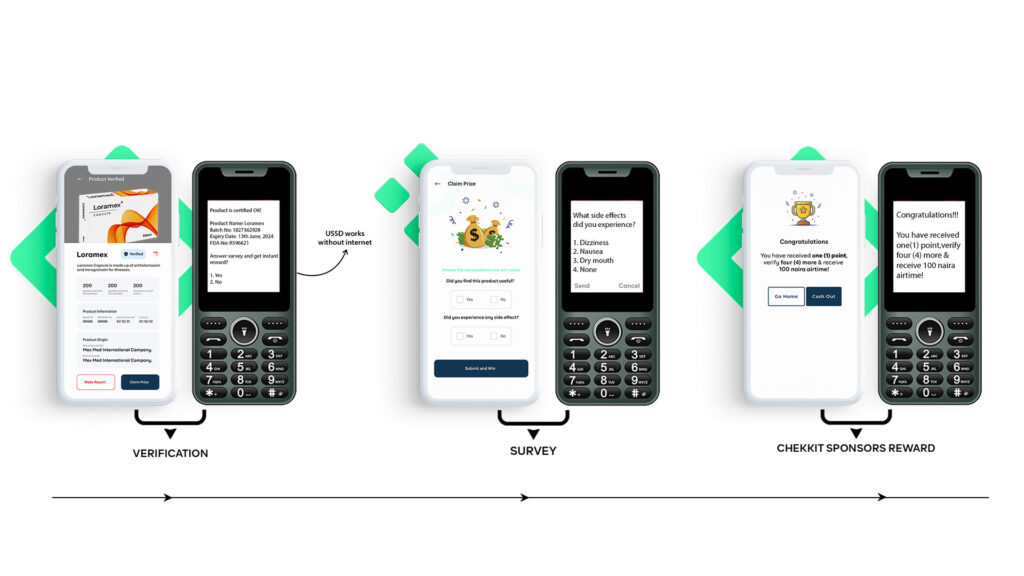
Serialization helps in the verification and identification of counterfeit medicines by allowing patients and healthcare workers to check the authenticity of a drug before use. By scanning the code with a mobile phone or a handheld device, they can verify that the medicine is genuine and has not been tampered with.
An extra benefit of verifications using Chekkit is the insights on things like side effects, drug efficacy, patient behaviours, and general last-mile feedback that can be collected directly from patients when they verify products.
How Does End-To-End Supply Chain Traceability Help To Fight Counterfeit Drugs?
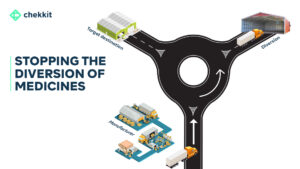
In an inefficient supply chain, there may be a lack of control over the movement and storage of goods. This can make it easier for counterfeiters to introduce fake products into the system. These weak links, such as poorly managed warehouses or transportation systems are vulnerable to exploitation by counterfeiters.
For example, a poorly secured warehouse can be broken into, and counterfeit products can be introduced into the supply chain. Finally, the lack of visibility into the movement of goods makes it difficult to detect the presence of counterfeit products that might have entered one way or the other.
By using the serialization already implemented on the packaging of medicines, you can monitor your products as they change hands through the supply chain, establishing a clear chain of custody. This visibility and the verifications at the different supply chain points will make it very difficult for counterfeit products to move through the chain without being detected.
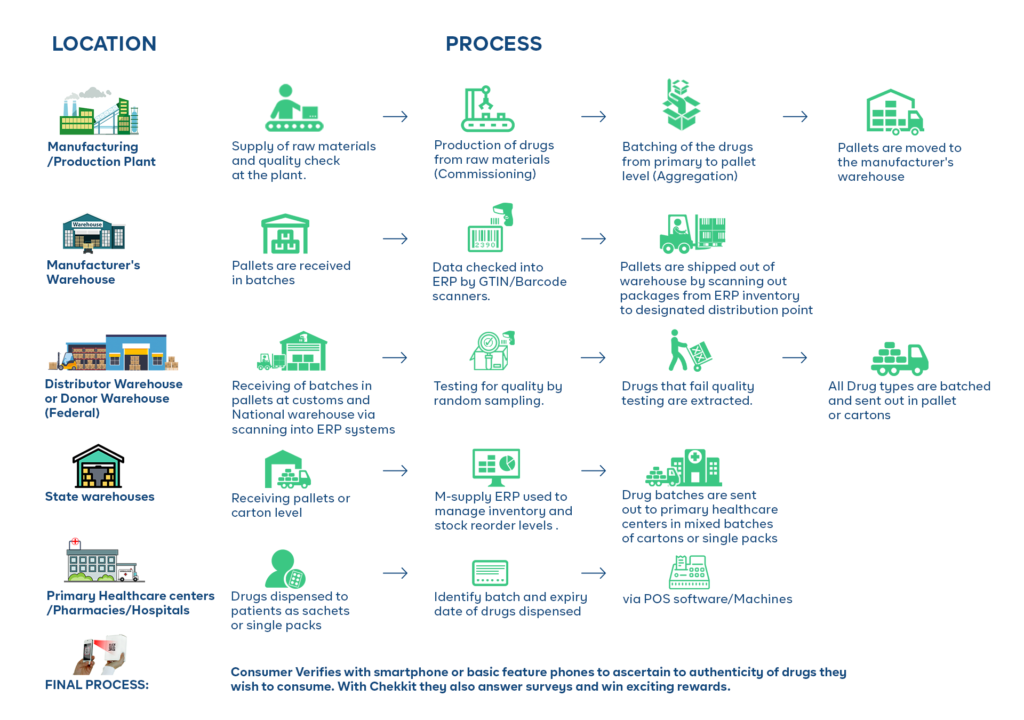
An extra benefit of implementing traceability using Chekkit’s infrastructure is that you don’t have to change your current production or operations processes. If you already have a solution that generates and manages serial numbers for your products, you can integrate with Chekkit and use the codes to connect your entire supply chain. What about distributors, wholesalers, and warehouses? Chekkit can also integrate with the inventory management or fulfillment solutions they already use. This makes connecting your entire supply chain to provide visibility super easy and less challenging.
What Are The Roles of Stakeholders in Fighting Counterfeit Drugs with Serialization & Traceability?
While every stakeholder across the entire supply chain has different roles to play, we have streamlined it to the 5 most important players if we’re to see significant progress in eliminating counterfeit drugs from shelves of pharmacies across Africa. They are:
Manufacturers: It is their role to ensure that their products are serialized straight from the factory. Here are the 2 major ways of implementing serialization.
Regulators: Arguably the most important of the lot. They have to pass and enforce laws that mandate traceability. They manage and monitor the database of products and movements that happen within their countries.
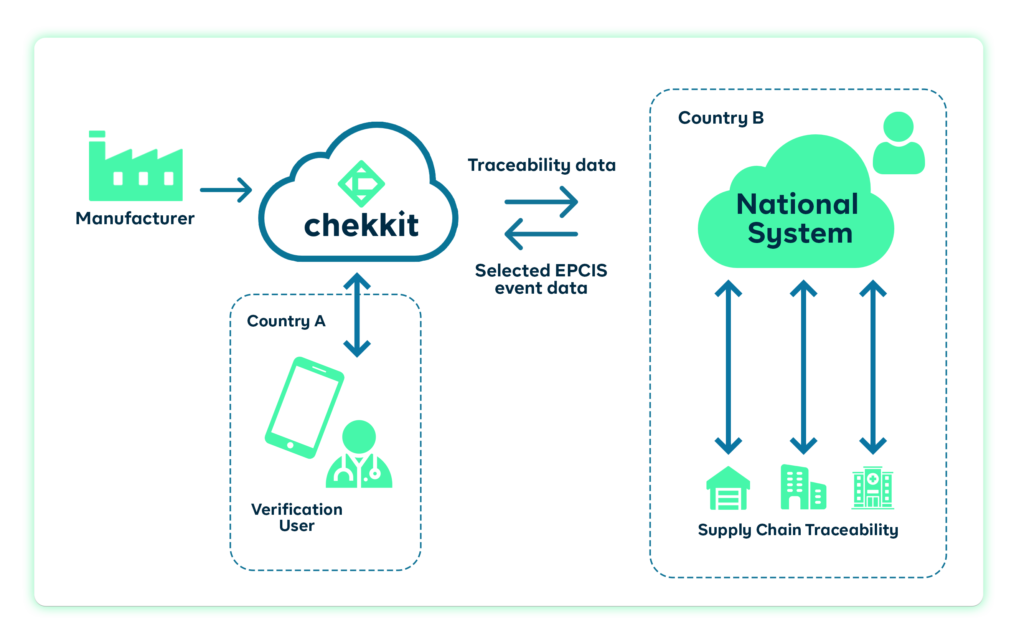
Donors & NGOs: As one of the biggest importers & distributors of medicines in Africa, donor bodies need to spearhead the onboarding of their federal & state warehouses as well as health centers that dispense donated medicines.
GS1: Provide the standards that are used in serializing products and exchanging data between stakeholders in the pharma supply chain. They work closely with regulators to implement these standards.
Traceability Solution Providers: They build the actual software that all the stakeholders actually interface with. They build their software using the guidelines provided by GS1 and integrate it with the regulatory database of different countries.
In conclusion, while the problem of counterfeit medicines remains a significant issue in many African countries, there has been significant progress made in enforcing traceability as many regulators are stepping up their efforts in partnership with GS1 and its solution providers.
What should be expected over the next few years? It will definitely get much harder for criminals to distribute counterfeit drugs and Chekkit is proud to be one of the solution providers spearheading the effort.
If you’re interested in learning more about how our traceability solutions can help you stop counterfeit drugs for good, feel free to request a demo of our traceability software today!
As a pharmaceutical manufacturer, importer, distributor, regulator, or donor, this technology could help you secure the distribution of medicines and ensure that only the right products reach patients safely and efficiently.