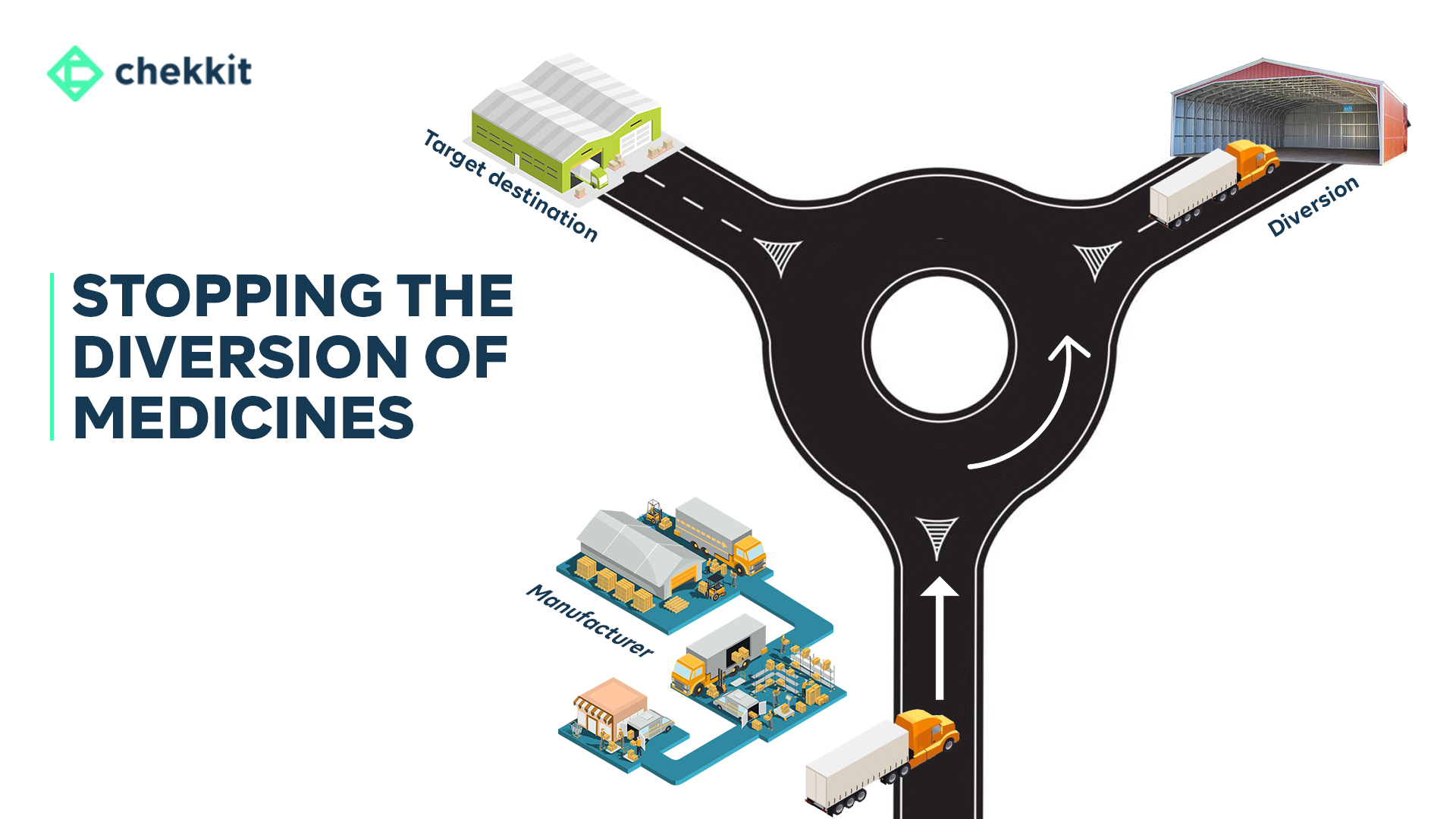
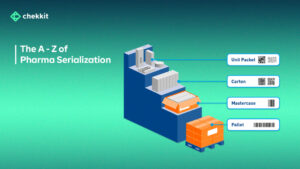
What is Market Diversion of Medicines?
Market diversion refers to the unauthorized distribution of pharmaceutical products from their intended market. This can occur when products meant for a specific geographic region are instead sold in another market, either at a higher or lower price.
This can cause a bunch of problems. The medicine might not be stored in the right conditions, the expiration date might have passed, or even worse, it could be fake medicine. All of this can be dangerous for the people taking it, not to mention it’s a real bummer for the drug company’s bottom line as it undermines their pricing strategies and brand reputation.
Thanks to the internet, it’s now easier than ever for people to sell medicine in places it wasn’t meant to go, making market diversions a common problem in the pharmaceutical industry.
But, don’t worry, there are solutions out there to help fight against it and keep medicine flowing where it’s needed. One such method is the use of a traceability system allowing for product verifications.
How Does Traceability Stop The Market Diversion of Medicines?
1. Identifying Market Diversions in Real-time
By using technologies such as radio-frequency identification (RFID) tags, 2d barcoding, and product verification, manufacturers can monitor the movement of their products and see where exactly these products are turning up.
With Chekkit’s traceability software (ChekTrace), for example, RFID tags can be attached to pallets and cartons so you can monitor if and when products leave the warehouse with minimal human input. If a product is detected outside of an authorized channel, the manufacturer can immediately take action to prevent it from reaching patients.
Lastly, the GS1 standard data-matrix or QR codes on your products, will also allow consumers to verify their medicines before use. This provides you with data on the location of the consumer and if they are using a product not meant for that location, they can easily be notified and the product tracked to identify where the breach happened.
Working with chekkit means you can monitor this data, get alerts, and take corrective action right from your ChekTrace dashboard
2. Creating a Tightly Regulated Ecosystem
Mandating traceability throughout the supply chain will help manufacturers, and most importantly, regulators to ensure that all products are properly monitored, reducing the risk of market diversions. These types of regulations will mandate all supply chain stakeholders to verify products and establish a clear chain of custody.
In addition, by having detailed records of the movement of each product, it’s easier to catch and prosecute those who engage in market diversions. This can serve as a deterrent to others and help create a culture of compliance, reducing the risk of market diversions in the future.
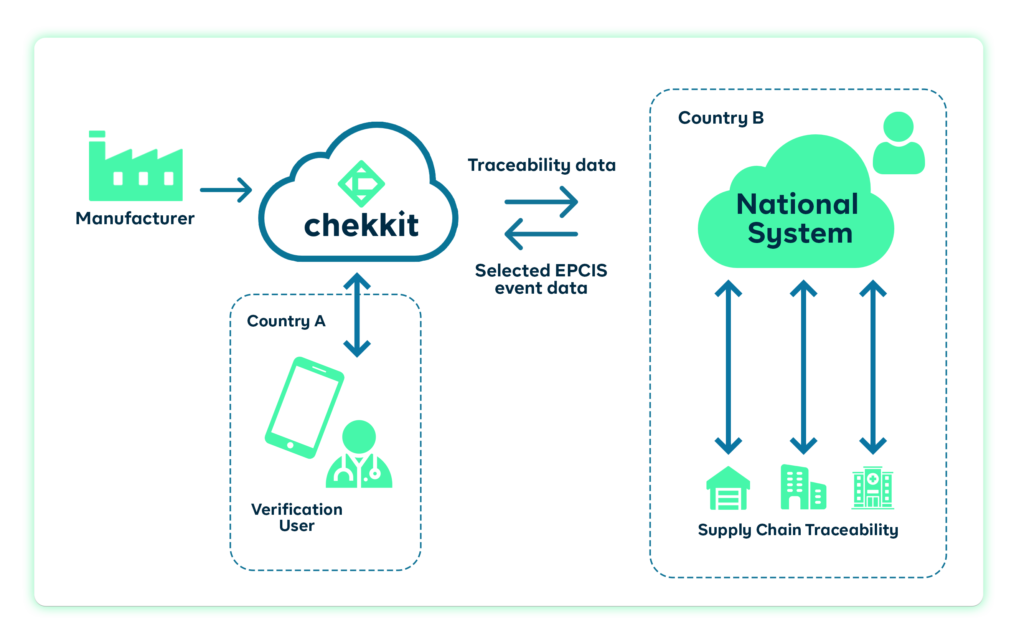
Currently, many countries mandate the use of the GS1 EPCIS standard in serializing the packaging of medicines to enhance traceability. As a GS1 solution provider, Chekkit has worked closely with governments in Afghanistan, Nigeria, etc to help their regulators implement end-to-end traceability using the GS1 standard.
3. Rewarding Good Behaviour
Finally, traceability solutions can be used to reward good behavior throughout the supply chain. By using the data generated by traceability solutions, manufacturers can identify those distributors, wholesalers, and retailers who always follow best practices, encouraging others to follow suit.
For example, manufacturers could offer discounts, priority shipping, or other incentives to those who consistently comply with best practices. This can help create a culture of compliance, as those who follow the rules will be rewarded for their efforts.
you can choose from the different default rewards available on Chekkit as a means to reward your supply chain partners or create custom rewards of your own. These rewards can then be distributed automatically and tied to specific good behaviours with respect to them following your traceability guidelines.
In conclusion, there are several ways you can implement traceability depending on the level of sophistication of your production operations but before you choose any solution, make sure you read our article on the 5 most important criteria to consider.
If you’re interested in learning more about how traceability solutions can help you stop market diversions, feel free to request a demo of our traceability software today!
As a pharmaceutical manufacturer, supply chain manager, or logistics expert, this technology could help you stay ahead of the curve and ensure that your products reach patients safely and efficiently.