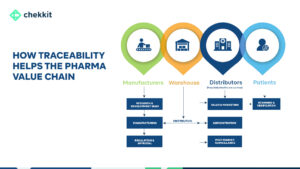
There’s a lot that goes on from when a drug is manufactured to when it gets into the hands of the patient that needs it. If GS1 standards didn’t exist today, that whole process would be a nightmare.
Granted, medicine distribution is still not where it should be in terms of security and efficiency, but the progress made thus far is because of the work that GS1 does as a non-profit organisation.
A Brief History of GS1
Back in 1969, the US retailing industry was having serious issues with the time it took people to check out at stores. They decided to create a special committee to look into it and in 1974, the first-ever product with a barcode was scanned.
In 2004, the Global Data Synchronization Network (GDSN), a global, internet-based initiative that enables trading partners to efficiently exchange product master data was established. The GDSN was a collaborative effort between the European Article Numbering Association (EAN) and the Uniform Code Council (UCC) of the USA.
By 2005, after being used in over 90 countries, the name GS1 started getting used as a means to reinforce its messaging of one global system of standards. Over the years GS1 standards have been used in different industries from healthcare to logistics and retail with a presence in over 100 countries.
For this article, we will be focusing on its application in the pharmaceutical industry.
What is the GS1 EPCIS Standard?
The GS1 EPCIS (Electronic Product Code Information Services) is GS1’s flagship data sharing standard for enabling visibility, within organisations as well as across an entire supply chain of trading partners and other stakeholders.
It helps provide the “what, when, where, why, and how” of products and other assets, enabling the capture and sharing of interoperable information about status, location, movement, and chain of custody.
What: The “What” refers to the physical or logical object that is being tracked, such as a pallet of goods or a shipment of products.
When: The “When” refers to the point in time when an event related to the object occurs, such as when the object is produced, shipped, or received. This information is used to track the movement of the object over time.
Where: The “Where” refers to the physical location of the object, such as a warehouse or a retail store. This information is used to track the movement of the object through different locations.
Why: The “Why” refers to the reason for the event, such as the business process or purpose for the movement of the object.
How: The “How” refers to the method used to track the object, such as RFID tags, barcodes, or other identification methods.
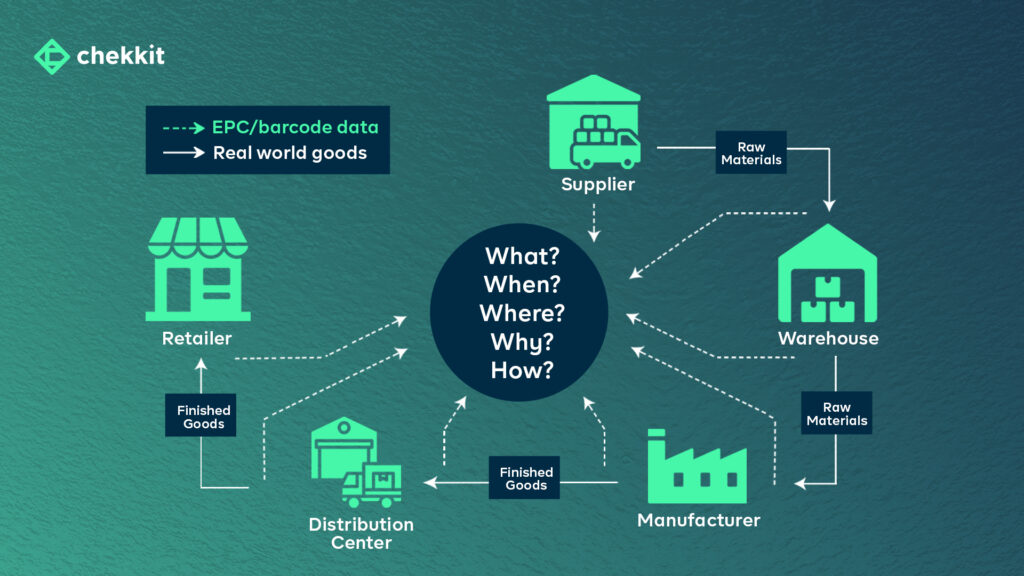
By capturing and tracking these five elements, the GS1 EPCIS system provides a comprehensive view of an object’s journey through the supply chain, including where it has been, when it was there, why it was moved, and how it was tracked. This information can be used to improve supply chain visibility, optimize logistics and reduce costs, and ensure compliance with regulations.
How Does The GS1 EPCIS Work For Pharmaceutical Traceability?
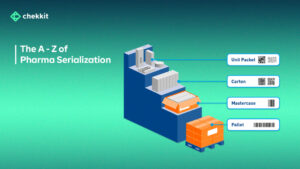
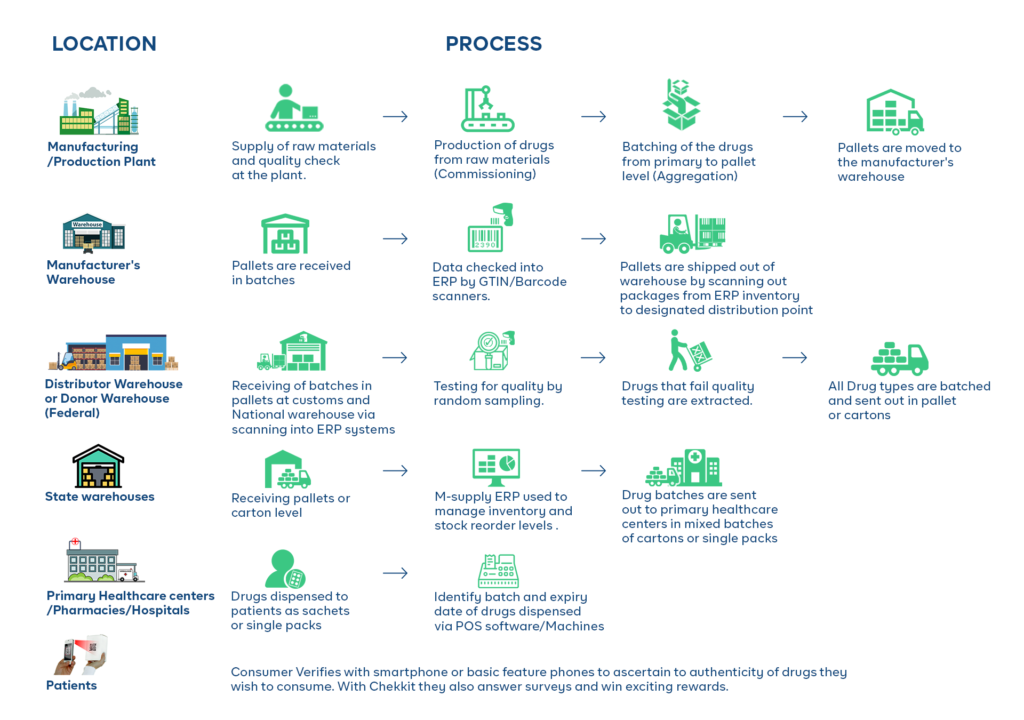
For pharmaceuticals, this information is stored on unique bar codes on the different packaging levels and is updated as the product moves through the supply chain.

The GS1 EPCIS standard defines several event types that can be used to capture and track different types of information related to a pharmaceutical product’s journey through the supply chain. These standard event types include:
Object Creation Event (Commissioning): This event type is used to capture information about when an object is first created and enters the supply chain, such as when a batch of pharmaceuticals is produced.
Object Event: This event type is used to capture information about when an object is moved, such as when a batch of pharmaceuticals is shipped from a manufacturing facility to a warehouse.
Aggregation Event: This event type is used to capture information about when an object is combined with other objects, such as when several small batches of pharmaceuticals are combined into a larger batch.
- Disaggregation Event: This is the opposite of the Aggregation event. It is used to capture information about when an object is broken down into smaller parts or components. For example, when a large batch of pharmaceuticals is broken down into smaller batches for distribution to different locations.
Transformation Event: This event type is used to capture information about when an object is transformed or changed in some way, such as when a batch of pharmaceuticals is repackaged or relabeled.
Inspection Event: This event type is used to capture information about when an object is inspected, such as when a batch of pharmaceuticals is inspected by a regulatory agency.
Disposition Event: This event type is used to capture information about when an object is disposed of, such as when a batch of pharmaceuticals is destroyed because it has expired or has been recalled.
Why is The GS1 EPCIS Standard Needed?
The GS1 EPCIS system can provide several benefits to the pharmaceutical industry, including:
Improved supply chain visibility: By capturing and tracking information about the movement of pharmaceuticals through the supply chain, the GS1 EPCIS system can help to improve visibility into the location and status of pharmaceuticals at all times. This can help to ensure that the correct batches of pharmaceuticals are delivered to the correct locations, and that any issues with the batches can be traced back to their origin.
Optimized logistics and inventory management: By providing real-time information about the location and status of pharmaceuticals, the GS1 EPCIS system can help to optimize logistics and reduce costs. For example, it can help to ensure that the right amount of pharmaceuticals are delivered to the right place at the right time, reducing the risk of stockouts or overstocking.
Improved recall management: By recording all events of a product, the GS1 EPCIS system can provide a detailed traceability of a product from the manufacturing to the end consumer, which helps to quickly identify and isolate products in case of a recall and reduce the risk of product being exposed to the public.
Counterfeit detection: An example of this in practice is using the unique GS1 bar codes on medicine packages, which can be scanned at the point of sale to confirm that the medicine is genuine by either the drug store with it’s POS system or with by the patient with a phone.
How Can I Get My Products To Be EPCIS Compliant?
Because GS1’s focus is to create and maintain the standards, they don’t actually build the software that the manufacturers and supply chain stakeholders interface with. To implement the EPCIS standards, you will need to work with a GS1 solution provider.
GS1 solution providers are technology companies that have successfully integrated their software with the EPCIS database. Here’s a breakdown of most of the processes that you will need to go through to go LIVE with traceability in about 6 months or less.
Identify your traceability needs: Before working with a GS1 solution provider, you will need to determine what specific traceability needs you have for your pharmaceutical products, such as tracking products from the manufacturing facility to the point of sale, or identifying counterfeit products.
Research GS1 solution providers: Research different GS1 solution providers and compare their services, expertise and experience in the pharmaceutical industry.
Contact and evaluate providers: Contact potential providers and evaluate their qualifications and experience.
Select a provider: Once you have evaluated your options, select a provider that best meets your needs.
Implement the solution: The provider will work with you to implement the traceability solution, which may include assigning unique identifiers to your products, setting up a central database, and training employees on how to use the system.
Test the solution: Once the solution is implemented, test it to ensure that it is working correctly and that all stakeholders are able to access and update the information in the central database as needed.
Continuously monitor and improve: Continuously monitor the solution and work with your provider to make any necessary updates or improvements to ensure its continued effectiveness.
Compliance: Work with your provider to ensure that the solution is compliant with any relevant traceability regulations and standards, such as are in many countries across the globe.
Monitor and maintain your system: Your provider will help you to monitor and maintain the system, but it’s also important to periodically review the system’s performance, identify areas for improvements and make changes accordingly.
Chekkit is the first GS1 traceability solution provider in Nigeria and has been implementing traceability systems in Nigeria, India, Germany, Afghanistan, Pakistan, Ethiopia, to mention but a few.
Will you like to see how brands like Merck have used Chekkit to stop counterfeits & collect first-party patient data? Get a free product demo