The pharmaceutical industry is constantly evolving, and with each advancement, there is an increasing need for traceability in various stages of drug development and distribution.
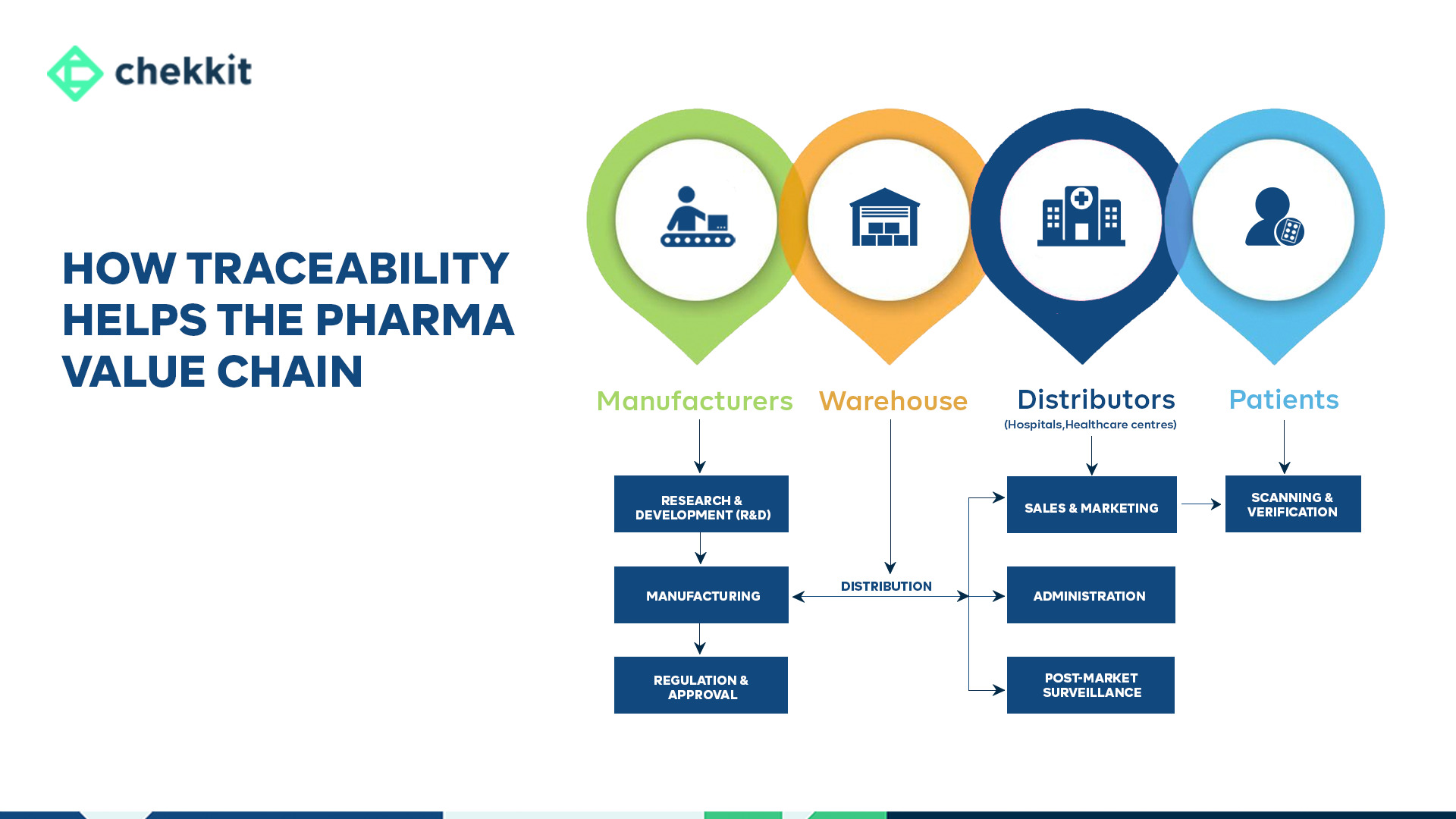
The pharmaceutical value chain refers to the series of interconnected stages that are involved in the development, production, and delivery of a pharmaceutical product from its origin to the end customer. It can be broken down into several key stages, from research & development all the way to post-market surveillance
What Are The Benefits of Traceability to the Typical Pharmaceutical Industry Value Chain?
Traceability refers to the ability to track the movement of a product from its origin to the end user. In this article, we will discuss the benefits of traceability in the 7 stages of the pharmaceutical industry value chain and how many manufacturers are already taking advantage to drop costs of production, and deliver superior quality medicines to patients.
1. Research & Development (R&D)
The drug development process begins with the identification of a therapeutic need and the subsequent development of a new drug through scientific research, laboratory testing, and clinical trials.
A traceability system allows for the tracking of the drug’s progression from laboratory testing to clinical trials, which can help identify any issues and enable necessary changes to be made promptly. This system helps reduce the risk of regulatory non-compliance and speeds up the drug development process.
Benefits of Chekkit's Traceability Solution in Pharma R&D
Whenever patients find a medicine that has been serialized by Chekkit (i.e it has the GS1 standard data matrix code or QR code on its packaging), they can verify it with their phone, take a short 3-question survey to provide feedback, and after doing that, win a reward.
The data collected from this process is then run through our machine learning model to help you understand side effects, drug efficacy, and then identify the not-so-glaring opportunities for new product development or improvements that can give you the edge over the competition. You can also find great and willing candidates for your trials from your regulatory-compliant patient database on Chekkit
2. Manufacturing:
Everyone understands the importance of producing drugs on a large scale to meet the demand of patients and the challenges that come with it. The processing, formulation, and packaging of these products require meticulous attention to detail to ensure that the final product is safe, effective, and compliant with regulatory standards.
Implementing a traceability system during the production process is vital to improve the quality control process and reduce the risk of product recalls. By tracking the production process and keeping detailed records, manufacturers can quickly identify and resolve any issues that may arise, such as product defects or contamination.
Benefits of Chekkit's Traceability Solution in Pharmaceutical Manufacturing
By choosing to on-board or work with only suppliers already on-boarded on Chekkit’s traceability platform, you can track the history of the APIs (Active Pharmaceutical Ingredients) and other raw materials used in the manufacturing process to be sure of their origin and processing.
Chekkit also keeps a digital history of every batch produced and by integrating with your production line, you have this data down to the second and it can be valuable in the event that a recall is needed due to contamination or defects.

3. Regulation & Approval:
Medicines are arguably one of the most regulated products globally. Because regulations are different based on location and these policies are changing quickly, it can become a very daunting process for both the regulators and manufacturers.
An end-to-end traceability solution can help to streamline the regulatory approval process and ensure regulatory compliance. The system can provide detailed information about the drug and its development process, which can be used to support regulatory submissions. This helps to ensure that the drug meets the necessary standards and requirements, reducing the risk of rejection or delay in the approval process.
Benefits of Chekkit's Traceability Solution in Regulating & Approving Medicines
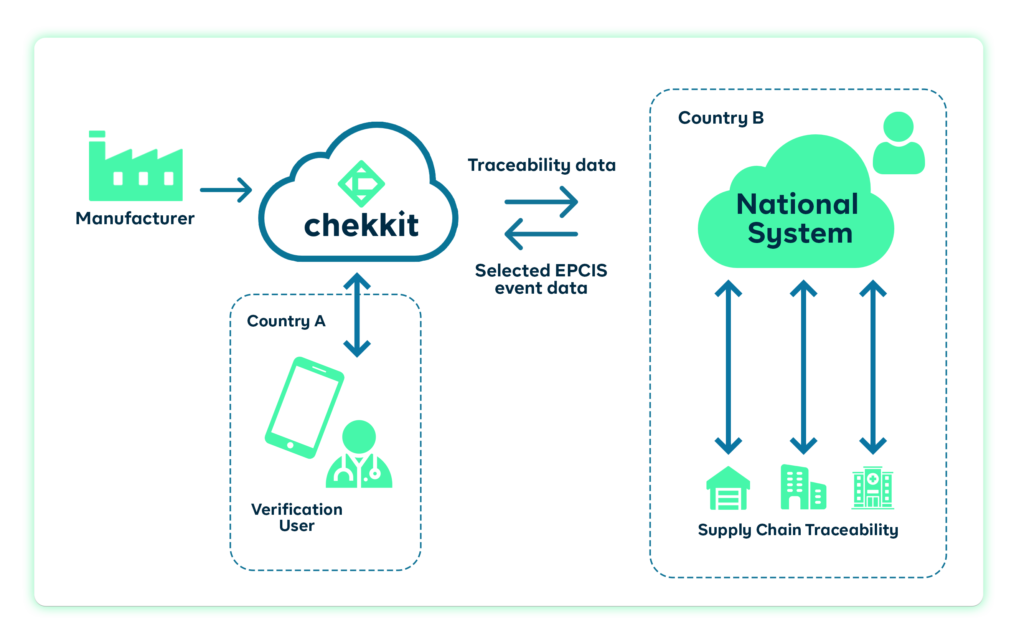
For regulators who don’t have a database or repository of the pharmaceutical products sold in their countries, they can build and maintain their repository on Chekkit’s traceability platform.
These repositories can then be connected directly to the manufacturer’s master data, giving regulators a 360-degree view of all products in their respective countries.
For countries with repositories and functioning systems, you can integrate our APIs to monitor products up to the last-mile patient. This can stop counterfeits for good and help penalize stakeholders who distribute sub-standard products.
4. Distribution:
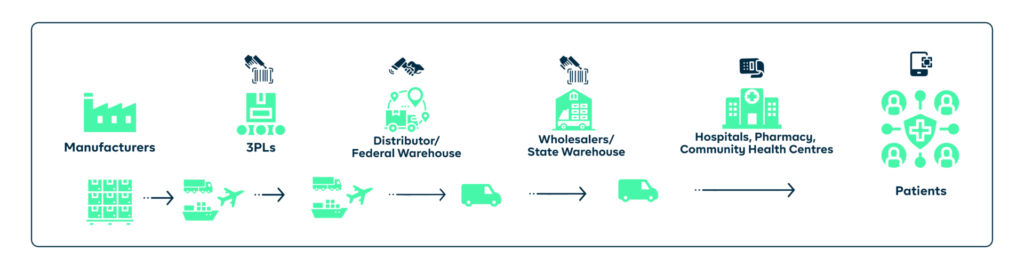
To be successful, it is crucial to recognise and address the challenges that come with drug distribution. These include the improper storage of drugs, inefficient and erratic delivery timing, counterfeit drugs, sabotage, and the logistical difficulties of reaching remote or underserved areas.
You will improve the efficiency of the drug distribution process and reduce the risk of product diversion by simply implementing a traceability solution. Tracking the movement of drugs from the manufacturer to the end user will help you identify and resolve any issues, such as theft, loss, or unauthorised distribution.
Benefits of Chekkit's Traceability Solution in Drug Distribution
Manage your entire GS1-standard barcoding system for all packaging levels (from pallets up to the medicine’s primary pack) as well as enable all stakeholders to verify & read these codes, you can monitor the scans as they happen through the distribution chain and see where every batch is at any time.
Your warehouses, distributors, and even healthcare providers can prevent stock-out and wastages from expired stock by using our inventory tools or connecting with whatever they already use. For essential medicines, real-time tracking can provide more information about things like temperature or pressure of your products as they are being transported.
5. Sales & Marketing:
To keep increasing sales, you must market your products excellently to healthcare providers and patients. This means better market research, product positioning, promotion, sales force training, and above all, a solid patient engagement strategy.
With a traceability system implemented, you get real-time data about product availability and sales. You can use these insights to optimise the sales and marketing strategies and spot new opportunities. The system can also help to identify and resolve any issues, such as product shortages or overstocking, which can negatively impact sales.
Benefits of Chekkit's Traceability Solution in Pharma Marketing
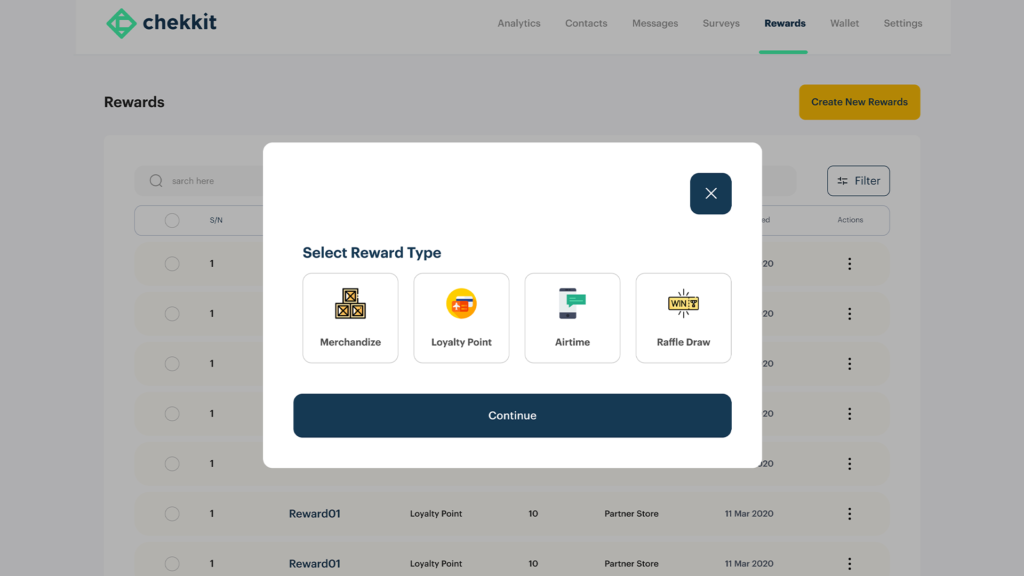
For brands who are looking to really become more patient-centric, Chekkit is that direct access to their consumers. Patients can scan your medicines to confirm their originality and get rewarded for answering up to 3 quick questions.
The insights from Chekkit not only show you clearly which locations sales are being made but can also reveal the demographics of your consumers, their habits, patterns, and needs. Run promotions for your OTC products and make sure the rewards are getting to the actual buyers and not being diverted.
6. Administration:
Establishing accurate dosing guidelines, ensuring proper patient adherence, preventing medication errors, and minimizing the risk of adverse side effects are some of the challenges we must overcome to ensure the safety and efficacy of drugs and maximize patient outcomes.
A traceability system will definitely improve the administration of drugs by healthcare professionals. The system can provide detailed information about the drug, including its composition, dosage, and administration instructions, which can be used to optimize the administration process. This helps to ensure that the drugs are administered correctly, reducing the risk of adverse reactions or treatment failure.
Benefits of Chekkit's Traceability Solution in Administering Medicines
Monitoring of medicines during dispensing is very critical for the success of any anti-counterfeit effort. By connecting the POS systems of pharmacies and healthcare centers to the government’s repository, the HCPs can confirm that what is being sold is authentic. It is the role of regulations to enforce HCPs to take this action for every medicine they sell.
For patient education, you can send follow-up messages via bulk SMS to patients who have verified your products before and start helping them live better and more productive lives. As an added layer of security, patients can also verify if the medicines they buy are fake or expired.
7. Post-Market Surveillance:
After the drug has been approved and is on the market, you must continue to monitor its safety and efficacy through various programs and studies. But because direct access to patients is basically non-existent for many stakeholders, getting access to the last-mile insights needed is tough.
A robust traceability system provides valuable tools for gathering real-time data regarding the safety and efficacy of drugs, thereby enabling stakeholders to identify any concerns and take prompt corrective action, such as updating labels or conducting recalls. As a result, the implementation of such a system is crucial for maintaining the integrity of drugs and ensuring that the health and safety of consumers are protected at all times.
Benefits of Chekkit's Traceability Solution in Post-Market Surveillance
The survey data collected from patients when they verify products can reveal things like possible side effects or adverse reactions to products. Our patient engagement can also be used to constantly communicate with patients and establish a 2-way flow of information.
Lastly, in the case of a recall, you can track individual batches and products directly from your ChekTrace dashboard.
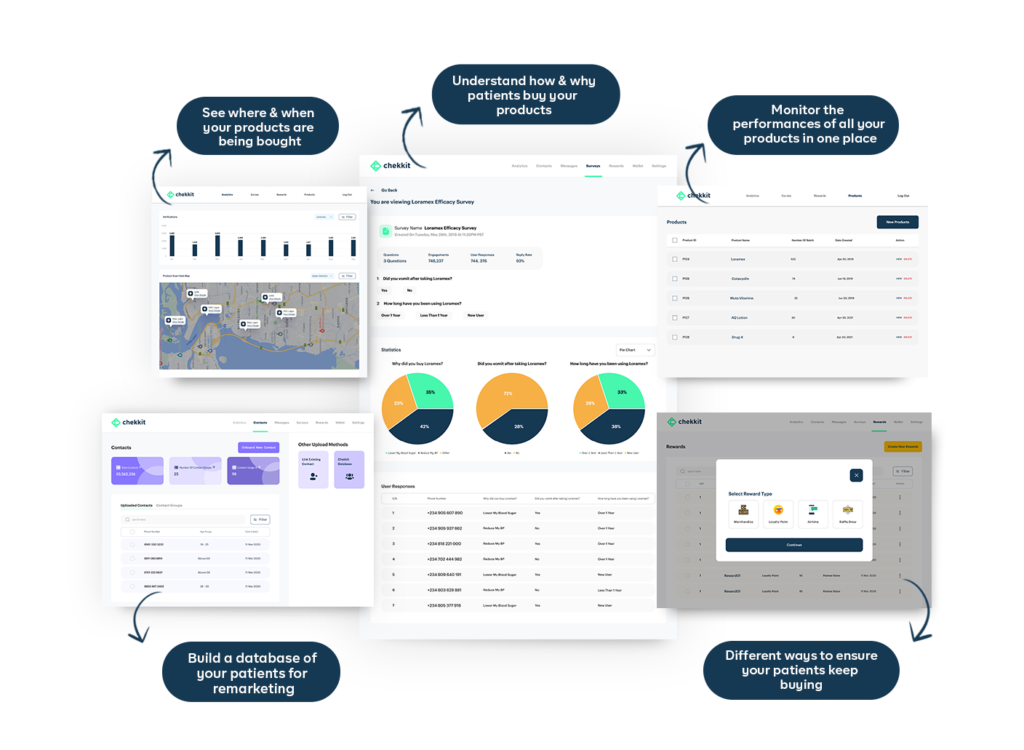
In conclusion, traceability plays a crucial role in ensuring the safety, quality, and efficiency of the pharmaceutical industry. From the R&D stage to sales and marketing, a traceability system can provide real-time data, improve quality control, and reduce the risk of product recalls and counterfeits. However, before you think of implementing a traceability solution, make sure you are working with a certified GS1 solution provider.
Are you looking for a traceability solution for your pharmaceutical company? Request a demo of Chekkit’s traceability solution today and see how it can help improve the efficiency and quality of your drug development and distribution process.