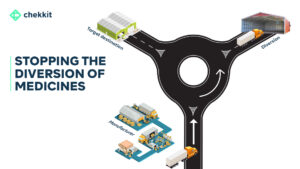
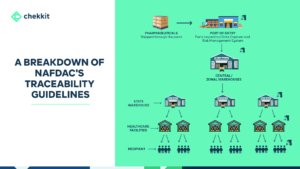
Over the next 24 months, the Nigerian pharmaceutical industry will experience a huge shift. Finally catching up with the rest of the world in mandating that pharmaceutical products implement end-to-end serialisation and traceability. The National Agency for Food and Drug Administration and Control (NAFDAC) has said this regulation will take effect from the end of 2024.
For Chekkit, this is a huge opportunity to scale its tech stack that provides manufacturers with serialisation and traceability of all packaging levels in line with global standards. Chekkit has raised an undisclosed round from Adaverse, a Cardano ecosystem accelerator, with participation from existing investors like RTA, HoaQ, Launch Africa, and Blockchain Founders Fund. This news is coming after Chekkit was officially announced as the first approved GS1/NAFDAC traceability solutions provider in Nigeria.
Vincent Li, founding partner at Adaverse, commented,
“Chekkit is not just another anti-counterfeiting solution. Rather, it aims to repair the prevailing rift in consumer-manufacturer trust with the blockchain-secured channel, prioritising consumer insights. We see the potential to transform the supply chain industry and disrupt the DataFi market and we’re excited to support the scaling of Chekkit’s infrastructure.
“Startups like Chekkit are well positioned to take advantage of the emerging data-driven economy in this digital age. With the building blocks of Web3, and the use of Cardano blockchain tools, Chekkit can help young economies leap ahead in data authenticity and gain valuable consumer insights.” says Shogo Ishida, co-CEO EMURGO Middle East and Africa
The raised funds will help Chekkit onboard more manufacturers across Nigeria and other regions in Africa, while also spreading its tentacles to new markets in India, UK and the Middle East with a target to become the #1 Traceability platform in Africa.
Reacting to the fundraise, here’s what Dare Odumade (Chekkit’s CEO) had to say;
“Since raising our pre-seed round of $500k in 2021, Chekkit has partnered and integrated its traceability and consumer intelligence solution with SAP (the global software giant) enabling pharmaceutical brands that already use SAP’s Advanced Track and Trace Platform to be able to collect and analyze last-mile patient data acquired via user surveys embedded in the GS1 serial codes (unique identifiers used for product verification). The company has also integrated the GS1 global standards system, making our serialisation software regulatory compliant in over 100 countries globally.
Funds will be used for scaling our team, tech and product distribution. We are expanding our team by hiring for more senior roles and launching in new countries like India and the UK. India because 70% of drugs sold in the MENA region are imported from India, while the UK gives us access to the Eurozone which is also responsible for producing a good number of chronic medications shipped to Africa.
We use the blockchain (distributed ledger) for securing the unique identifiers we generate for products or ingest from our ERP partners to prevent the duplication of serial codes and to also secure consumer personal data. In the near future, we will deploy an open source web3 API giving access to an ecosystem of 3rd party developers who wish to develop solutions that can leverage these drug/CPG product data for inventory, shopping, shipping, and payment solutions development”
Chekkit Technologies was founded in 2018 by Dare Odumade with the support of Oluwatosin Adelowo and Samuel Ukhueleigbe as Co-Founders. The 4-year-old startup has so far helped secure over 50 million pharmaceutical and consumer goods products, ensuring we the end users always have access to safe and genuine products.